
The survival of the human species in the face of a high number of incoming genetic mutations has remained an important problem in evolutionary biology. A newborn human is estimated to receive about 70 new mutations that the parents did not have. While these provide a source of novelty for the species, a large fraction of them can also be damaging, or likely to interfere with the biological function that a gene has for the organism. So how do we not mutate into oblivion?
A study published today in Science by Shamil Sunyaev and colleagues reports that as a species, humans get around this mutation problem thanks to certain interdependencies within the genome. Basically, if a new mutation occurs in a genome that already contains many damaging mutations, it has a stronger effect than if it occurred in a genome with just a few other damaging mutations. Thus, when a genome carries beyond a particular number of damaging mutations, it is significantly less likely to contribute progeny to the next generation.
Researchers including lead author Mashaal Sohail, PhD candidate in Systems Biology at HMS and member of the Sunyaev lab, studied population samples from Europe, Asia and Africa and found a significant depletion of individuals carrying a large number of highly damaging mutations overall. Their results suggests that natural selection against highly damaging genetic mutations is ongoing in humans, and that it is aided by synergistic interactions between different parts of the human genome, a form of epistasis. As selection due to pre-reproductive mortality is deeply relaxed in industrialized humans, it is likely that the observed signal comes from selection due to differential fertility, or pre-natal selection (only ~30% of human conceptions result in live births). This observation is general and is not limited to our own species. The same effect was independently observed in fruit flies.
The findings also help solve another long-standing riddle in evolutionary biology, that of the sustainability of sexual reproduction. Because sex shuffles two genomes together, it creates genomes with both very few and very many mutations every generation. If damaging mutations in a genome behave synergistically, genomes with a high number of damaging mutations are then very unlikely to leave progeny. Evolutionary theory, thus, holds that sexual reproducers may be more successful than asexual reproducers because they are able to purge multiple damaging mutations from the population with single genetic deaths post sex-induced genetic remixing. This is precisely what was observed in this study. By showing that sexual reproducers such as humans and fruit flies have a significant depletion of individuals with a large number of highly damaging mutations, the study provides support to theories that suggest that sex has an evolutionary advantage in a deterministic sense. That is to say, sex had to come about in a species such as our own to allow for more effective natural selection because the mutation rate is too high to sustain otherwise.
Sunyaev is both professor of biomedical informatics at HMS and professor of medicine at Brigham and Women’s Hospital. Collaborators included University of Michigan professor Alexey Kondrashov, the Institute for Information Transmission Problems (Kharkevich Institute) of the Russian Academy of Sciences in Russia, and the Department of Neurology and Neurosurgery at the University Medical Center Utrecht in the Netherlands.
Also See
Missing Mutations Suggest a Reason for Sex — Quanta Magazine
Making sense of survival — Harvard Gazette
Author Commentary
The Making of an Evolutionary Geneticist

The views and opinions expressed below are those of the author and do not necessarily reflect the official policy or position of the Department of Biomedical Informatics, Harvard Medical School, or Harvard University.
Not many people look at a beautiful rugged mountain landscape and think of genetics. Fortunately or unfortunately, I am one of them now. How did I get here? It all started with a meeting with my soon to-be advisor, Shamil Sunyaev, at Harvard Medical School. Other than telling me that he was Tatar and “culturally muslim,” he also drew me a picture and told me a story. The mutation rate in humans is too high he said—how do we survive? He drew a histogram and drew an arrow with a big capital U pointing at it. On this histogram of mutation burden per individual, the U (representing the per-genome rate of deleterious mutations) was referring to the fact that every generation a newborn human is estimated to receive ~70 new mutations that the parents did not have. While mutations provide a source of novelty, a large fraction of them can also be damaging for the individual and the species.
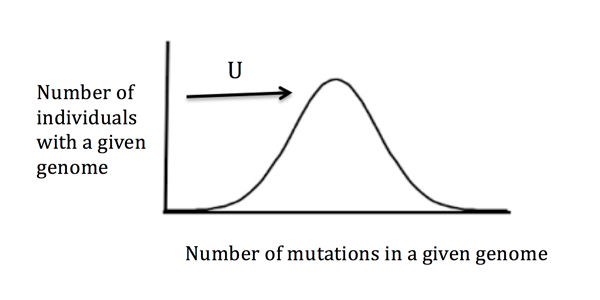
At the end of the meeting, he printed out two papers and handed them to me with a snide smile, “I know you went to MIT and all but if you can handle math...” His framing comment was well deserved. The papers were dense, difficult and theoretical. Written two decades apart, the papers were “Deleterious mutations and the evolution of sexual reproduction” written by one Alex Kondrashov in 1988 and “The mutational load with epistatic gene interactions in fitness” written by Motoo Kimura and Takeo Maruyama in 1966. I left the meeting a little perplexed. Shamil seemed earnestly puzzled by the fact that humans even survived. This was my first real introduction to how geneticists saw the world. I had never worked on human sciences before, and even the depiction of the human as a histogram was curious to me—it was an initiation into thinking of humans in population and species terms instead of in individual terms.

I enjoyed struggling with the challenge of these historical papers, and began a research rotation in Shamil’s lab. I also remember noticing that Alex’s paper was published just two days before I was born in 1988. Little did I know then that mutational load and evolution of sex, two concepts that I had happily lived without ever hearing about for the first 23 years of my life will come to occupy a large space in it for at least the next 5 years. Slowly, the problem and its constituents began to sink in. The survival of the human population in the face of a high number of incoming genetic mutations had been pondered on, starting in the 1950s, and remained an important problem in evolutionary biology. Theory such as Alex’s showed that, as a species, humans get around this problem through dependence between damaging mutations in the genome. Basically, if a new mutation occurs in a genome that already contains many damaging mutations, it has a stronger effect than if it occurred in a genome with just a few other damaging mutations. When a genome carries beyond a certain number of damaging mutations, it is significantly less likely to contribute progeny to the next generation. In this way, the population is able to purge multiple damaging mutations without as many individual deaths. The idea is such that what is good for the population is not necessarily what is good for the individual.
Further, it was becoming clear to me that the problem was intimately tied to another one that evolutionary biologists have struggled with in the last century – how is sexual reproduction so prevalent given that it seems to be the evolutionarily less successful strategy? In what we call the “two-fold cost of sex,” the problem is that sexual reproducers only pass on half their genome, while asexual reproducers pass on entire clones of themselves, and yet sex hardly ever leaves a population once it has entered it. The solution that has been proposed by many theorists went as follows.
Sex shuffles two genomes together, and in so doing, creates genomes with very few and very many mutations every generation. If damaging mutations in a genome behave synergistically, genomes with a high number of damaging mutations are then very unlikely to leave progeny. Evolutionary theory, thus, holds that sexual reproducers may be more successful than asexual reproducers because they are able to purge multiple damaging mutations from the population with single genetic deaths post sex-induced genetic remixing. Mutation is universal and this would provide sex with a deterministic evolutionary advantage. That is to say, sex had to come about in a species such as our own to allow for more effective natural selection because the mutation rate is too high to sustain otherwise. This goes back to the individual–species dichotomy from earlier. In Michael Turelli’s words, one could think of this process as Kondrashov’s hatchet falling on genomes that contain more than a certain number of mutations. Such genomes are recreated every generation by sex even if the mutations may have emerged on separate genomes at first. The population is better off—of course, the same cannot be said of the individuals under the hatchet, but such is natural selection.
At the other end of the project, I began to look at population samples from modern human populations. I had always leaned towards empiricism and liked the idea of using genomic data to see if I could test for evidence for such hatchet-like natural selection in humans. The Dutch Government was funding a project to sequence genomes of 250 healthy human trios. We had 88 individuals from their pilot study to start with. I started learning how to do “genomics”, albeit very slowly and with many crashes and burns of scripts and the lab computer cluster. Already, in the rotation in what felt like the most productive three weeks of my life, I had plots and what felt like a result. That was now 5 years ago. We looked at the rarest (or youngest) mutations that have appeared in the human species. These mutations are most likely to be damaging as natural selection weeds out “bad” mutations pretty fast so mutations that have stuck around a long time are usually neutral or benign. We used other biological information about the genome and its functionality to further pick from these mutations, the ones that are most likely to be damaging and the ones that are most likely to be neutral. We then counted these up for each individual and saw what looked like a depletion of individuals with a large number of damaging mutations compared to what you would expect by chance. Moreover, we saw no such effect for neutral mutations.
Bam! I was going to have a high-impact paper in three weeks. Life doesn’t quite work like that though. With the very thankful appearance of a summer student, Jean Fan, in the lab who began to work with me, we quickly realized that the depletion was not “natural selection” but rather a bug in my code. Regardless, the excitement that built up in those first three weeks gave enough momentum to the project to keep it going five more years. With this time came new and larger datasets of both European and non-European descent to study this effect in. Also came collaboration with a student of Alex Kondrashov’s, Olga Vakhrusheva, who successfully found the same signature of natural selection in the fruit fly. It also gave us time to think about and rule out all other possible aspects of technology and geography that may potentially lead to the same signature.
As we worked our way through the theory that had accumulated in the last century, we learned that there has been and continues to be another school of thought. This school pondered on the idea that sexual recombination may have come about randomly and then persisted. We ran many simulations, new calculations and re-looked at our data through this new potential lens before we finally convinced ourselves of three things. One, we see, overall, a depletion of individuals with a large number of damaging mutations in both human and fruit fly populations. We see no such effect for neutral mutations in either humans or fruit flies. Two, we think this is an observation of the hand of natural selection or the fall of Kondrashov’s hatchet on natural populations. This observation helps explain how humans, as a species, are able to survive despite our high mutation rate. It is a sacrifice of individual genomes for the benefit of the overall population genetic pool. Lastly, we think this observation also helps shed light on why sex may have an evolutionary advantage. By remixing genomes every generation, sex allows this process of natural selection against damaging mutations to happen so effectively. As another classical geneticist Muller put it, instead of losing one mutation with one “genetic death,” the population is rid of multiple mutations with single genetic deaths.
What of the mountain range though? Through working on this project, I also came to understand that interactions between different parts of the genome also have implications for evolutionary fate. Biologists have a special name for the kind of genetic interactions discussed throughout this post: epistasis. If different genes don’t act independently, but instead interact with each other, we say there is epistasis in the genome. People often think of terms like “survival of the fittest” when seeing the world through an evolutionary perspective—a single mountain peak we are all trying to climb at the expense of each other. If epistasis is present however, the number of available evolutionary paths becomes constrained. Just as if genes were instead events in your life, the more different events in your life were actually dependent on each other, rather than being independent, the more constrained the final outcome of your life will actually be. Similarly epistasis, or dependence between different genes, leads to ruggedness in the (fitness) “landscape” that organisms such as ourselves are trying to traverse. Instead of a single mountain peak then, it may instead be more like the mountain range in the beginning of this post with many local niches and few global optimums. Another way of looking at this project then is as a small jab to try to help reveal the fitness landscape which natural biological beings, in particular humans and fruit flies, are flowing through.
—Mashaal Sohail is a PhD candidate in Systems Biology at HMS and lead author of "Negative selection in humans and fruit flies involves synergistic epistasis," published today in Science.