
The COVID-19 pandemic has reshaped health and medicine in ways both dramatic and subtle. Some of the less obvious shifts can only emerge from analysis of millions of pieces of data—patient records, medical notes, clinical encounter reports.
Taken in isolation these data points may offer tantalizing anecdotes. Analyzed together, they can offer a bird’s-eye view of interesting interplays and reveal important trends, giving clinicians and public health experts valuable clues that can inform both prevention and intervention.
Marinka Zitnik, assistant professor of biomedical informatics in the Blavatnik Institute at Harvard Medical School, uses data science and machine-learning methods to glean such insights—hiding in plain view—about disease development and progression, therapeutic outcomes and response to treatment.
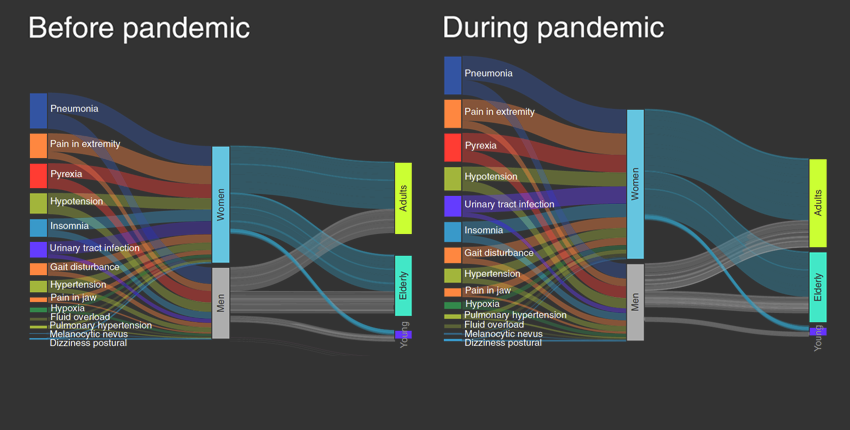
Zitnik’s latest research, a study published Oct. 5 in Nature Computational Science, analyzes patterns in adverse medication events before and during the pandemic.
In the study, Zitnik and co-authors Xiang Zhang, a post-doctoral research fellow at HMS, and Marissa Sumathipala, a graduate researcher at Harvard University, used more than 1.4 million medical reports involving 2,821 drugs.
The researchers found that 54 types of adverse events increased in frequency during the pandemic, even though overall, the number of adverse medication events went down somewhat. Furthermore, the analysis revealed telltale gender and age differences in the likelihood of adverse events.
Read full article in HMS News