UDN Scales Up
Coordinating Center at DBMI funded five more years as new clinical sites and research facilities added nationally
Coordinating Center at DBMI funded five more years as new clinical sites and research facilities added nationally
Grants to improve and accelerate the diagnosis of rare and undiagnosed conditions were made to academic medical centers across the nation Monday. The new awards are part of the second phase of the National Institutes of Health’s Undiagnosed Diseases Network (UDN). The total investment planned for the UDN over the next four years will be approximately $100 million, pending the availability of funds.
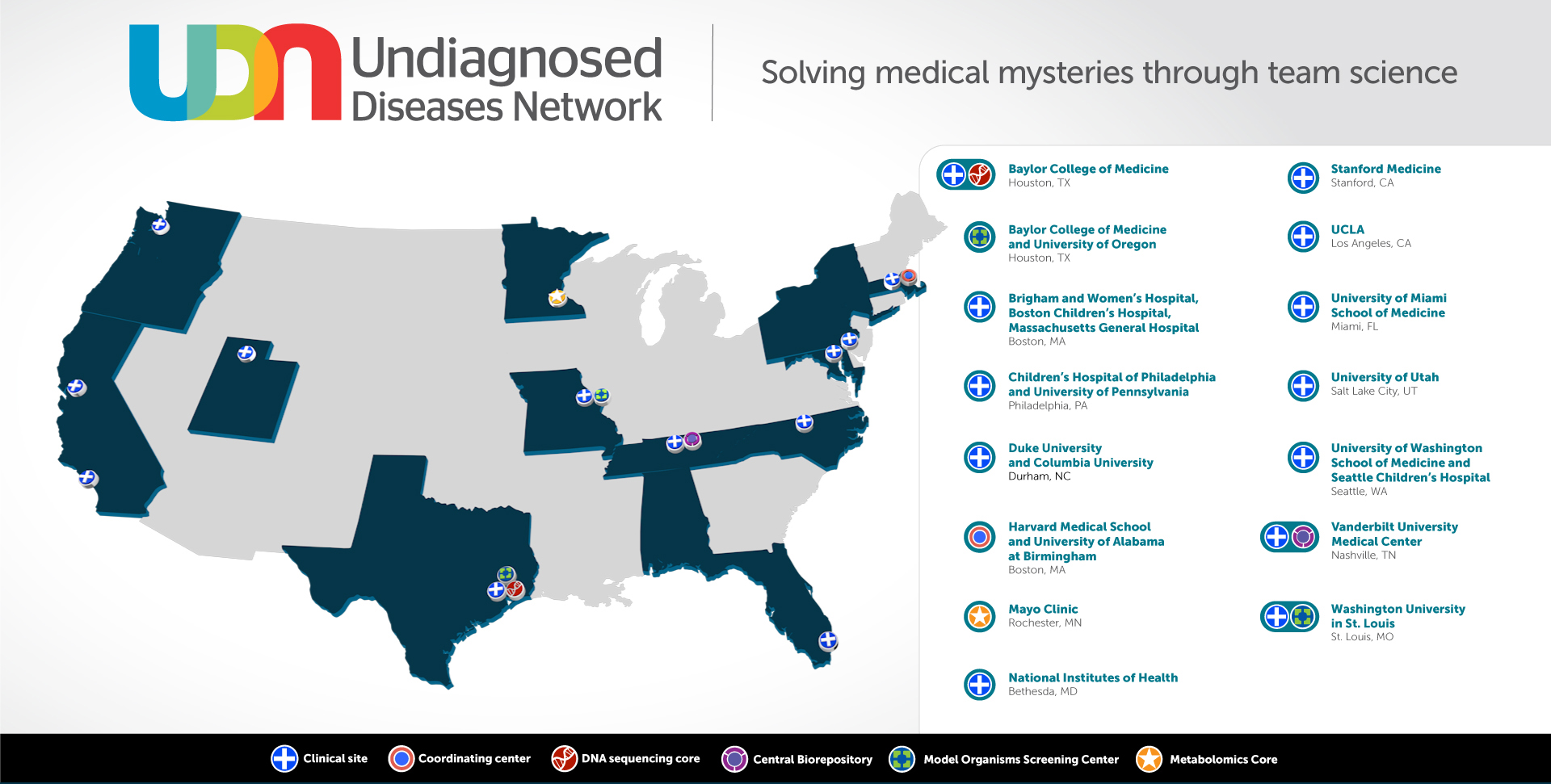
These grants will expand the UDN from seven to 12 clinical sites, increasing the geographical distribution of the nationwide network and the number of people with access to a UDN clinical site. Since opening to applications in 2015, the network has already diagnosed over 200 cases that had long been mysteries to the medical community.
“The UDN is pioneering a new personalized medicine model for helping those patients who have historically been the most difficult for the medical community to diagnose,” said James M. Anderson, M.D., Ph.D., director of NIH’s Division of Program Coordination, Planning, and Strategic Initiatives (DPCPSI), which provides financial support and joint leadership for the network via the NIH Common Fund. “By bringing together a nationwide network of top clinicians and laboratory researchers using the most up to date medical technology and knowledge, the UDN is able to provide hope to these patients, and in many cases discover a diagnosis.”
The UDN was launched to build on the success of the Undiagnosed Diseases Program (UDP) at the NIH Clinical Center. “The UDN takes advantage of cutting-edge technologies such as genomic sequencing, metabolomics and assessing patient variants in model organisms to give clinicians new, powerful information to help understand the cause of extremely rare diseases,” said Anastasia L. Wise, Ph.D., program director for the UDN in the National Human Genome Research Institute’s Division of Genomic Medicine.
Five new clinical sites will join the existing six academic clinical sites at Baylor College of Medicine, Houston; Duke University, Durham, North Carolina and Columbia University, New York City; Brigham and Women’s Hospital, Boston Children’s Hospital, and Massachusetts General Hospital at Harvard Medical School; Stanford Medicine, California; the University of California Los Angeles; Vanderbilt University Medical Center, Nashville, Tennessee; and the NIH UDP, Bethesda, Maryland from Phase I to make up Phase II of the UDN. The five new clinical sites are:
In addition to the new clinical sites, new research cores will be part of Phase II of the UDN. A new Metabolomics Core at the Mayo Clinic, Rochester, Minnesota, has been awarded (Principal Investigators: Ian Lanza, Ph.D. and Devin Oglesbee, Ph.D.) providing untargeted metabolomics and targeted biomarker analyses, and a new Model Organisms Screening Center at Washington University in St. Louis, (Principal Investigators: Lilianna Solnica-Krezel, Ph.D. and Tim Schedl, Ph.D.) increasing the zebrafish modeling capacity and adding C. elegans as a new model system for the UDN.
Phase II of the UDN will also retain the coordinating center at Harvard Medical School and University of Alabama at Birmingham, the genome sequencing core at Baylor College of Medicine, and the model organism screening center at Baylor College of Medicine and the University of Oregon, Eugene. A complete list of funded awards can be found at https://commonfund.nih.gov/Diseases/fundedresearch.
The UDN awards are managed by NHGRI, the National Center for Advancing Translational Sciences, and the National Institute of Neurological Disorders and Stroke.
For more information about the UDN application process, visit https://undiagnosed.hms.harvard.edu/apply/
For more information about the UDN program, visit https://commonfund.nih.gov/Diseases.
About the NIH Common Fund: The NIH Common Fund encourages collaboration and supports a series of exceptionally high-impact, trans-NIH programs. Common Fund programs are managed by the Office of Strategic Coordination in the Division of Program Coordination, Planning, and Strategic Initiatives in the NIH Office of the Director in partnership with the NIH Institutes, Centers, and Offices. More information is available at the Common Fund website: https://commonfund.nih.gov.
About the National Center for Advancing Translational Sciences (NCATS): NCATS conducts and supports research on the science and operation of translation — the process by which interventions to improve health are developed and implemented — to allow more treatments to get to more patients more quickly. For more information about how NCATS is improving health through smarter science, visit https://ncats.nih.gov.
About the National Human Genome Research Institute (NHGRI): NHGRI is the driving force for advancing genomics research at the National Institutes of Health. By conducting and funding world-class genomics research, training the next generation of genomics experts, and collaborating with diverse communities, NHGRI accelerates scientific and medical breakthroughs that improve human health. Learn more at genome.gov.
About the National Institute of Neurological Disorders and Stroke (NINDS): NINDS is the nation’s leading funder of research on the brain and nervous system. The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
© 2025 by the President and Fellows of Harvard College